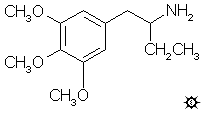
#1. AEM
alpha-ETHYLMESCALINE; 2-AMINO-1-(3,4,5-TRIMETHOXYPHENYL)BUTANE; 1-(3,4,5-TRIMETHOXYPHENYL)-2-AMINOBUTANE
SYNTHESIS: To a solution of 45 g 3,4,5-trimethoxybenzaldehyde in 1.2 L
IPA, there was added 125 g nitropropane and 67.5 g t-butylammonium
acetate and the reaction mixture was held at reflux for 16 h. This
was poured into 6 L H2O, and extracted with 2x250 mL hexane. The
pooled extracts were stripped of solvent under vacuum giving a residue
that slowly set to a crystalline mass. On filtering, there was
obtained 9.4 g of a crude yellow product which, on recrystallization
from hexane provided 8.7 g of slightly sticky bright yellow crystals
of 2-nitro-1-(3,4,5-trimethoxyphenyl)butene-1, with a mp of 71-73 °C.
A second recrystallization from hexane gave fine yellow crystals with
a mp of 72-73 °C. Attempts at the preparation of this nitrostyrene by
the more conventional methods with ammonium acetate in acetic acid led
either to the formation of a white product C23H30N2O8 which was
composed of a molecule of the nitrostyrene, one of the benzaldehyde
itself, and a molecule of ammonia, or to 3,4,5-trimethoxybenzonitrile,
from reaction with the decomposition products of nitropropane.
A stirred suspension of 5.9 g LAH in 310 mL anhydrous Et2O was held at
a gentle reflux in an inert atmosphere. A solution of 8.5 g
2-nitro-1-(3,4,5-trimethoxyphenyl)butene-1 in 125 mL Et2O is added
drop-wise over the course of 0.5 h. The reaction was maintained at
reflux for 6 h, then cooled, and the excess hydride destroyed by the
cautious addition of 300 mL 1.8 N H2SO4. The phases were separated,
and the aqueous phase brought to a pH of 6 by the addition of a
saturated Na2CO3 solution. The neutral solution was brought to a
boil, and clarified by filtration through paper. To the hot filtrate
there was added a solution of 8.9 g picric acid in 100 mL boiling
EtOH. The mixture was stirred and cooled, with the formation of a
heavy yellow crystalline mass. After standing in the ice tub for
several hours the mixture was filtered, providing 8.0 g of the picrate
salt with a mp of 176-181 °C from H2O. A solution of this salt in 300
mL boiling H2O was treated with 60 mL concentrated HCl. On cooling,
there was a deposition of picric acid, which was removed by
filtration. The aqueous filtrate was washed with 3x50 mL
nitrobenzene, then with 3x50 mL Et2O. The pH was brought above 9 by
the addition of aqueous NaOH, and the filtrate was extracted with
3x100 mL CH2Cl2. Removal of the solvent from the pooled extracts gave
a nearly colorless oil, which was dissolved in 300 mL anhydrous Et2O
and saturated with hydrogen chloride gas. The white crystals of
2-amino-1-(3,4,5-trimethoxyphenyl)butane hydrochloride (AEM) were
removed by filtration, Et2O washed, and air dried. They weighed 4.72
g.
DOSAGE: greater than 220 mg.
DURATION: unknown.
EXTENSIONS AND COMMENTARY: The extension of the two-carbon chain of
mescaline by alpha-methylation to the three carbon chain of TMA
approximately doubled the potency of the compound. And it was felt to
be a completely logical possibility that, by extending it one more
carbon atom, to the four carbon chain of alpha-ethyl-mescaline, it
might double again. And following that logical progression, the
doubling of potency with each additional carbon atom, the factor would
be 2 to the 7th power by the alpha-octyl (or 256x that of mescaline,
or a milligram as active dose) and with a side chain of a 70-carbon
alkyl group (alpha-heptacontylmescaline) it would take just a single
molecule to be intoxicating. This was rich fantasy stuff. As an
active compound, just where would it go in the brain? With an
80-carbon side-chain, would one-thousandth of a single molecule be
enough for a person? Or might a single molecule intoxicate a thousand
people? And how long a chain on the alpha-position might be
sufficient that, by merely writing down the structure on a piece of
paper, you would get high? Maybe just conceiving the structure in
your mind would do it. That is, after all, the way of homeopathy.
Maybe it was just as well that this added two-carbon side-chain with
lowered activity was already enough to disprove the doubling pattern.
But by the time this non-activity had been learned, the alpha series
had already been pushed out quite aways. The machinery of making the
appropriate nitroalkane was straightforward, by reaction of the alkyl
halide with nitrous acid, and separating the unwanted nitrite ester
from the wanted nitroalkane by fractional distillation. The
nitrostyrenes all formed reasonably although often in terrible yields,
and reduced reasonably, and all formed crystalline picrates for
isolation and crystalline hydrochloride salts for pharmacological
manipulation. But since the first of these, AEM, was not active,
there was no enthusiasm for tasting anything higher. This family was
never published; why publish presumably inactive and thus
uninteresting material? The Table presents the properties of the
precursor nitrostyrenes, and the product picrate and hydrochloride
salts, at least whatever information I can still find after thirty
years:
TABLE. Physical Properties of the alpha-Alkylmescaline Homologues and
their Precursor Nitrostyrenes
Code Name NS mp °C picrate mp °C HCl mp °C
APM Alpha-propylmescaline 82-83 214-218
ABM Alpha-butylmescaline 73-74 169-174 182-184
AAM Alpha-amylmescaline 54-55 162-163 155-158
AHM Alpha-hexylmescaline 51-52
ASM* Alpha-heptylmescaline 43-44
AOM Alpha-octylmescaline **
ANM Alpha-nonylmescaline 46-47 ***
AUM Alpha-undecylmescaline ***
* S is for septyl, to distinguish heptyl from hexyl. **Never
made, as no nonylbromide could be located to make the needed
nitrononane. ***The synthesis got as far as the nitrostyrene stage
when the inactivity of AEM was determined, and the project was
dropped.
| HTML and Design by Lamont Granquist & Erowid |
Used by Erowid with permission of author |