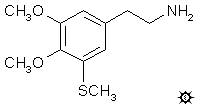
#155 3-TM
3-THIOMESCALINE; 3,4-DIMETHOXY-5-METHYLTHIOPHENETHYLAMINE
SYNTHESIS: To an ice cold and well stirred solution of 15 g vanillin
and 20 g sodium thiocyanate in 150 mL acetic acid there was added,
dropwise over the course of 15 min, a solution of 16 g elemental
bromine in 40 mL acetic acid. This was followed by the addition of 30
mL of 5% HCl and 300 mL EtOH, and stirring was continued for an
additional 30 min. The mixture was heated to its boiling point, and
filtered while hot. The mother liquor was diluted with an equal
volume of H2O, which initiated the crystallization of crude
5-formyl-7-methoxy-2-oxo-1,3-benzoxathiole as a flocculant yellow
solid. On filtration and air-drying, this weighed 12.5 g. After
recrystallization from EtOH, the product was white and had a mp of 164
°C sharp.
A suspension of 12.5 g of crude
5-formyl-7-methoxy-2-oxo-1,3-benzoxathiole in 100 mL MeOH containing
28.4 g methyl iodide was treated with a solution of 12 g NaOH in 100
mL warm MeOH. The mixture was held at reflux for 1 h and then the
solvents were removed under vacuum. A solution of 14.2 g methyl
iodide in 100 mL DMSO was added and the mixture stirred for 1 h. An
additional 2.4 g of NaOH and 16 g methyl iodide were added, and the
stirring was continued for another 2 h. The reaction mixture was
poured into 800 mL H2O, acidified with HCl, and extracted with 3x75 mL
CH2Cl2. The pooled extracts were washed with 5% NaOH, then water, and
the solvent removed under vacuum. Distillation at 110-130 °C at 0.4
mm/Hg gave 0.9 g 3,4-dimethoxy-5-(methylthio)benzaldehyde which had a
mp of 57-58 °C after crystallization from EtOH. Anal. (C10H12O3S)
C,H.
A solution of 0.9 g 3,4-dimethoxy-5-(methylthio)benzaldehyde in 100 mL
nitromethane containing 0.5 g anhydrous ammonium acetate was held at
reflux for 4 h. The excess nitromethane was removed under vacuum, and
the deep brown residue was dissolved in 4 mL hot MeOH. On cooling,
the yellow crystals were removed by filtration, washed with cold MeOH
and air dried yielding 0.4 g yellow crystals of
3,4-dimethoxy-5-methoxy-beta-nitrostyrene, with a mp of 119.5-120.5 °C
after recrystallization from EtOH. Anal. (C11H13NO4S) C,H.
To a solution of 1.0 g LAH in 25 mL anhydrous THF under He, cooled to
0 °C and vigorously stirred, there was added, dropwise, 0.7 mL of 100%
H2SO4, followed by a solution of 0.7 g
3,4-dimethoxy-5-methylthio-beta-nitrostyrene in 10 mL anhydrous THF. The
mixture was brought briefly to a reflux, cooled again, and the excess
hydride destroyed with H2O in THF, followed by the dropwise addition
of 15% NaOH until the solids became white and granular. The solids
were removed by filtration, the filter cake washed with THF, the
mother liquor and filtrates combined, diluted with an equal volume of
Et2O, and extracted with 2x40 mL dilute H2SO4. The aqueous extracts
were combined, washed with Et2O, made basic with aqueous NaOH, and
extracted with 2x50 mL CH2Cl2. The solvent was removed from these
extracts and the residue distilled to provide 0.4 g of a white oil
boiling at 124-130 °C at 0.2 mm/Hg. This oil was dissolved in 8 mL
IPA, neutralized with concentrated HCl, and diluted with 30 mL
anhydrous Et2O. The white crystalline product was the monohydrate of
3,4-dimethoxy-5-methylthiophenethylamine hydrochloride (3-TM) which
melted at 167-168 °C and weighed 0.29 g. Anal. (C11H18ClNO2SaH2O)
C,H,N.
DOSAGE: 60 - 100 mg.
DURATION: 8 - 12 h.
QUALITATIVE COMMENTS: (with 80 mg) I went into the experience with
the question of whether it (3-TM) might be a writing aid. I found a
considerable color enhancement (this was at the one hour point) and
there seems to be no problem in writing physical words. But there is
no urge to, as there are no new things. This is progressing into
something more complex and there is an interesting shielding effect.
I still have the desire to write and I sense that many things are
going on underneath, but my conscious control suppresses their
availability. It is now the third hour. Music. I would like to try
this material at 100 milligrams. Now awareness seems much more
pointed. I have need to build a writing table. This material is
physically relaxing, insisting repose, but with conflicting energy.
Seated in a chair, but I seem unable to find a comfortable position in
order to write.
Pine trees seem a good place
To start. Notwithstanding this table
Of pine, unfinished, unruled,
The pulp upon which we reveal
The unnerved thoughts.
How casual we are at discarding
Our feelings, a rubble we
Leave behind for the living.
Who among us can absorb
The spiritual load we see as
What others carry.
This material is not poetic, I should say, does not enhance poetry,
prose is much more comfortable. I think I should let the experience
develop further. It is now the fifth hour. There is something of a
violence (emotional) suppressed in all of us, a socially repressed
vision of oneself in a direct conflict with oneself. The music has a
lot to do with this material. And it changes with time. In the first
part there is sublimity, peacefulness, mild intoxication. And a lot
more tension in the part that followed the four hour point. There the
territories seem much better defined, with the benign shielding of the
first half largely dissipated. I have developed a slightly irritated
view of myself, probably wanting once again to regain the serenity.
(with 80 mg) Delightful day. Not insight depth but persistent
feeling of pleasant good humor. It is good-natured and very verbal.
Everyone talked and the instinct was to express and comment on
everything. There were no visuals during the first three to four
hours--with the eyes open one could barely detect the intoxication.
Eyes closed--quiet lovely window, no images. About +2. And then
someone brought in a radio with music on, into the room. There was a
tremendous eruption of closed-eyes visual images and fantasy. Bright
colors, funny, rich and elaborate. Marvelous. I was suddenly at +3.
Next day, no hangover. Pleasant feeling persisted.
(with 100 mg) I found the day had two halves. The first few hours
were characterized by occasional defensiveness (paranoia) and
irritability. In interpersonal interactions there was a guardedness,
due to a feeling of vulnerability. I went off by myself, and with
eyes closed, there was rich imagery and color synthesis to musical
imput. And then things smoothed out, and I could express an easy flow
of ideas and concepts without always watching my step. And then all
too soon, the intensity of the experience began fading away.
EXTENSIONS AND COMMENTARY: The amphetamine which would correspond with
this base would be 3,4-dimethoxy-5-methylthioamphetamine (3-T-TMA) and
should be an active compound. Its synthesis should be straightforward
from the benzaldehyde described above, employing nitroethane rather
than nitromethane. It is apparently an unknown compound.
| HTML and Design by Lamont Granquist & Erowid |
Used by Erowid with permission of author |