Originally published in (eds. Edward. J. Cone, Ph.D., Michael. J. Welch, Ph.D., and M. Beth Grigson Babecki, M.A.) Hair Testing for Drugs of Abuse: International Research on Standards and Technology, 1995, p. 91-120. NIH Publication No. 95-3727.
Gary L. Henderson, Martha R. Harkey, and Reese T. Jones
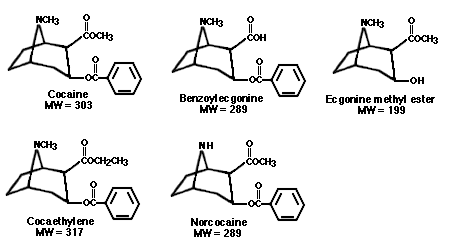
The use of hair as a specimen to detect cocaine use was first reported in 1981 (Arnold and Püschel 1981; Valente et al. 1981). In those studies, hair samples from suspected drug abusers were analyzed by radioimmunoassay (RIA) for the cocaine metabolite benzoylecgonine (BZE) in an attempt to verify a history of cocaine use. Additional studies using RIA followed shortly thereafter (Arnold and Püschel 1981; Valente et al. 1981; Baumgartner et 1982; Smith and Liu 1986; Michalodimitrakis 1987). The first gas chromatography/mass spectrometry (GC/MS) procedures for detecting cocaine in hair were not reported until 1987 (Balabanova and Homoki 1987). When this more specific technique was used, it was found that cocaine, not BZE, is the primary analyte in hair. The metabolites BZE and ecgonine methyl ester (EME), shown in figure 1, are present in such low and variable concentrations that they may result from environmental degradation of cocaine already present in hair. Because of the lack of specificity used in some of the early studies, it is difficult to evaluate much of this literature. For example, some investigators quantitated "cocaine" in hair using RIA antibodies highly specific for BZE, whereas others used RlAs more specific for cocaine. In addition, some digestion or extraction procedures could have caused degradation of cocaine.

FIGURE 1. Chemical structure and molecular weight (MW) of cocaine
and the metabolites
benzoylecgonine, ecgonine methyl ester, cocaethylene, and
norcocaine.
The past few years have seen the development of highly specific and sensitive GC/MS methods for the simultaneous detection and quantitation of cocaine and several of its metabolites in hair. These methods have been applied to hair samples obtained from a variety of animal species and human subjects. It is now clear from these more detailed studies that in all species studied and by all routes of administration, cocaine is incorporated into hair as the parent drug and can be detected in hair for months after administration. What has not been determined is the relationship between the dose and the amount of drug in hair or whether the position of the drug along the hair shaft can be used to determine the time of drug ingestion.
This chapter reviews results from the many laboratories throughout the world that are now engaged in hair analysis and compares them with the authors' controlled-dose studies involving human subjects.
Several analytical methods are used for detecting and quantitating cocaine and its metabolites in hair, but none has emerged as an accepted standard. Typically, each procedure includes the following steps: specimen collection, sample washing, digestion or extraction of the hair sample, Immunoassay screening, and confirmation or quantitation of the various analytes.
The greatest differences among the methods are in the sample preparation steps, that is, washing and digesting or extracting the hair sample. The authors used an elaborate method that incorporated "soft" digestion of the hair specimen to prevent any conversion of cocaine to BZE and solid-phase extraction of the digest so that relatively clean mass spectra were obtained for quantitating the low picogram amounts of drug found in their clinical studies (Harkey et al. 1991). The same methods have been used throughout so that results with various subjects studied during the past few years could be compared. Other laboratories have developed more rapid methods in which the samples were extracted with acid or organic solvent (Cone et al. 1991; Pirozhkov et al. 1992).
The site and technique for collecting the hair specimens are important because considerable differences in trace element and drug concentrations have been reported for hair samples collected from different sites (Chatt and Katz 1988, pp. 14-16). Morphine concentrations have been found to be highest in pubic hair, followed by axillary hair, then scalp hair (Kintz et al. 1993); methadone concentrations have been found to be highest in axillary hair, followed by pubic hair. then scalp hair (Balabanova and Wolf 1989).
Hair samples analyzed for cocaine are most typically obtained from the scalp. This site may be more susceptible to external contamination, but it also can be sampled less intrusively than the axillary or pubic regions. The posterior vertex region of the scalp is generally chosen as a sampling site because most hair in this area (85 percent) is in the active growing phase and thus more likely to incorporate drug. An adequate sample for testing, approximately 100 mg of hair, can be obtained by gripping a bundle of hair with the circumference of a pencil and then pulling gently to remove any loose strands of hair in the resting stage that are easily shed. The remaining strands are grasped firmly while the hair is cut as close to the scalp as possible. After cutting the hair, it is important to keep the root ends of the bundle aligned so that the hair strands can be cut accurately for subsequent segmental analysis.
Segmental analysis often is used in hair analysis in an attempt to correlate time of ingestion with location of drug along the hair shaft. In this procedure, the root ends of the hair sample are aligned, and the sample is cut into segments that can be analyzed individually. Typically, the sample is cut into 1-cm segments with each segment corresponding to approximately 1 month's growth, assuming a hair growth rate of 1 cm/month. However, the length of the segments can be varied, and some laboratories have found that cutting the hair specimen into shorter segments (e.g., 2 mm) results in better resolution (Uematsu et al. 1993).
Laboratory workers routinely wash the hair sample before analysis to remove lipids, oils, cosmetics, and any adhering drug. However, the efficacy of washing procedures in removing cocaine deposited on hair from external sources is controversial. Baumgartner and associates report that in most instances washing the hair specimen (5 to 10 mg) with 1 mL methanol for 15 minutes at 37 °C follow by three 30-minute washes with phosphate buffer (pH 6) at 37 °C removed any externally bound drug (Baumgartner 1989; Baumgartner and Hill 1992, pp 577-597). In some cases they found that more extensive washing was required and proposed a "kinetic wash criteria" --in which the amount of drug found in the various washes was compared with the amount of drug in the washed, extracted hair specimen-- to distinguish between passive exposure and active ingestion. Koren and colleagues (1992a) also found that washing removed all drug from a variety of hair samples that had been contaminated from externally applied cocaine. Their assay method, like that of Baumgartner, was RIA.
Other investigators have found that, despite extensive washing, enough residual cocaine remained on an externally contaminated hair sample to produce a positive test. Blank and Kidwell (1993) exposed control hair to radiolabeled cocaine in solution and found that washing removed most, but not all, the externally applied cocaine. They concluded that they were not able to distinguish passively exposed samples from actively exposed samples using a kinetic wash criterion similar to the one proposed by Baumgartner and colleagues (1982). In addition, they found that washing removed cocaine metabolites as well and suggested that extensive washing with solvents might more aptly be called extraction (Blank and Kidwell 1993). In fact, methanol is the solvent used most often in washing procedures and is the solvent used by many for extracting cocaine from hair (see below). Kidwell (1993) recommended using pentane as a washing solvent because it removes surface oils from hair but does not extract drug from hair.
The authors also found that extensive washing removed most but not all, of the cocaine deposited on hair either from "crack smoke" or from cocaine in an aqueous solution. For example, control drug-free hair was incubated for 72 hours in an aqueous solution of radiolabeled cocaine (4-3H-cocaine, specific activity of 14.77 mCi/mg) at concentrations of 15 ng/mL and 1 mg/mL. The treated hair was washed extensively with various solutions (distilled water, acetone, chloroform, methanol), and significant amounts of drug remained in the hair even though no drug was detected in the final wash solutions. Figure 2 shows a typical uptake and washout curve for human hair incubated in a cocaine solution and washed exhaustively in distilled water (Henderson et al. 1993). Large amounts of cocaine were incorporated into hair, and repeated washings removed only about 80 percent of the drug. Similarly, when control hair was placed in an enclosed 4-cubic-foot acrylic chamber and exposed to 10 mg cocaine base that was vaporized by heating it to 200 °C, the hair was found to contain cocaine concentrations that were greater than 100 ng/mg before washing. Vigorous washing removed most (as much as 95 percent), but not all, of the externally applied drug. Washing also reduced the amount of BZE and EME in hair samples from chronic cocaine users (Henderson et al. 1992), which suggests that washing may remove internally incorporated drug as well as that present from external contamination.
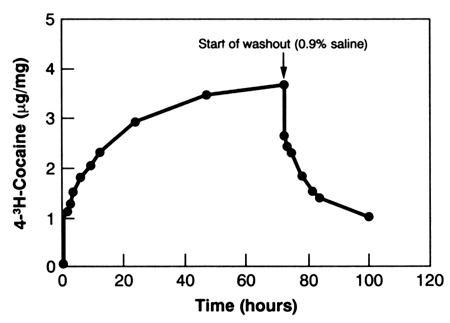
FIGURE 2. Uptake of 4-3H-cocaine into hair from a 1mg/mL bathing solution followed by washout from a 0.9-percent saline solution. Each data point is the mean value of duplicate 10-mg samples.
A variety of methods are used for the digestion of hair and extraction of incorporated drug. The most efficient method of destroying the protein matrix is alkaline digestion (incubation in 1 N NaOH for 1 hour at 100 °C); however, this method cannot be used for cocaine analysis because it completely degrades cocaine. For the simultaneous quantitation of cocaine, BZE, and EME in hair, it is preferable to use either enzyme digestion or extraction with acid or solvent.
Acid Extraction. Extracting hair with mineral acid (hydrochloric acid [HCl] or H2SO4) is an efficient method for extracting cocaine and metabolites from hair, and only a small amount of cocaine is converted to BZE with this technique (Cone et al. 1991). A method used by Valente and colleagues (1981), Balabanova and coworkers (1987), and Balabanova and Homoki (1987) called for pulverizing approximately 50 mg hair, then incubating in 0. 1 M HCI overnight at 45 °C. The acid extract was neutralized with 100 µL 1 M NaOH then diluted with phosphate buffer (pH 7.4) up to 2 mL. Martinez and colleagues (1993) used a similar procedure and incubated whole, unpulverized hair samples with 1 mL 0. 1 M HCI for 18 hours at 37 °C. Cone and colleagues (1991) used H2SO4 (1 mL, 0.05 M) in their extraction procedure and reported a recovery of 90 percent of the cocaine added to control hair with a 10- percent conversion to BZE.
Solvent Extraction. Methanol appears to be the solvent of choice for extracting cocaine and metabolites from hair. Pirozhkov and colleagues (1992) found that incubating washed hair samples (50 to 100 mg) in 3 mL methanol for only 2 hours at 60 °C yielded a recovery equivalent to acid extraction for 18 hours. Extending incubation period to 4 hours did not appear to increase recovery. The extracts were purified by a differential pH liquid-liquid extraction procedure similar to that for the extraction of cocaine from plasma (Jatlow 1975, pp. 133-137). No recovery data were given; however, a limit of detection for cocaine of 0.2 ng/mg hair was reported.
Graham and colleagues (1989) described a similar extraction procedure for BZE in which washed hair samples (2 mg) were sonicated with 1 ml methanol of 30 minutes and then incubated overnight at 45 °C.
Enzyme Digestion. The authors used a soft digestion technique for hair samples similar to that reported by Gill and coworkers (1985) but modified it to improve the recovery of cocaine and reduce chemical background in the GC/MS analysis (Harkey et al. 1991). Approximately 10 mg hair was placed in a screw- capped silanized glass centrifuge tube (10 mm wide x 100 mm deep) with 2.6 mL digest buffer (1 mL 1 M Tris HCI buffer, 20 mL 10 percent sodium dodecyl sulfate, and 79 mL deionized water) and with 0.4 mL 0 4 M dithiothreitol in 10 mM sodium acetate buffer and then was vortexed and incubated for 2 hours at 40 °C. Then 55 µL. proteinase K solution (10 mg/mL or 136 units/mL) was added; the sample was vortexed again and incubated overnight at 40 °C. phase extraction was used to isolate cocaine, BZE, and EME the digested hair samples.
Möller and colleagues (1992) described a faster procedure in which 20 to 30 mg hair was pulverized and then digested for 2 hours at 40 °C in a solution containing 75 µL -glucuronidase-aryl-sulfatase in 2 mL phosphate buffer (pH 7.6). They also used solid-phase extraction prior to derivatization and GC/MS analysis.
Both liquid-liquid extraction and solid-phase extraction have been used to purify hair extracts prior to analysis.
Liquid-Liquid Extraction. Pirozhkov and colleagues (1992) described a differential pH liquid-liquid extraction procedure for purifying hair extracts that is similar to a procedure for extracting cocaine from plasma (Jatlow 1975, pp. 133-137). Methanol hair extracts were evaporated under N2 at 40 °C; the residue was dissolved in 2 mL 0.1 N HCI and then was extracted with 4 mL hexane containing 1 percent isoamylol. After the residue was centrifuged for 5 minutes at 2,500 rpm, the hexane layer was discarded, and the acid phase was made alkaline to pH 9.2 by adding 0.03 mL NH40H and 0.5 mL 10 percent K2PO4 and then was extracted with 4 mL hexane-isoamylol mixture. The organic phase was evaporated under N2, and the residue was dissolved in methanol for subsequent analysis.
Solid-Phase Extraction. Möller and colleagues (1992) isolated cocaine and its metabolites from digested hair samples using Chromabond C18 extraction columns previously conditioned with 6 mL methanol and 3 mL water (H2O). After the hair digest was added, the column was washed with 3 mL H2O, 3 mL 0.25 N acetic acid, and then 3 mL H2O. The column then was dried by passing air through it for 10 minutes and centrifuging at 4,000 rpm for 15 minutes. Absorbed drugs were eluted three times with 500 µL of three parts acetone to one part dichloromethane.
In the authors' laboratory, cocaine and metabolites were extracted from the digested hair samples using "double mechanism" (reversed phase and ion exchange) extraction columns (Bond Elut Certify). After the columns were conditioned with 2 mL methanol and 2 mL 0.1 M phosphate buffer, the hair digest was added to the columns and the columns were rinsed with 3 mL deionized water, 3 mL 0. 1 M HCI, and 8 mL methanol. Cocaine, BZE, and EME then were eluted with two additions of 2 mL methylene chloride:isopropyl alcohol (4:1) with 2 percent ammonium hydroxide. The extracts were evaporated under N2 at 40 °C prior to derivatization and GC/MS analysis (Harkey et al. 1991).
RIA is the most popular screening method for hair analysis, probably because of its sensitivity and the availability of commercial reagent kits. Because drugs and their metabolites are present in hair in ng/mg or pg/mg concentrations, it is likely that only immunoassays that use radiolabeled or chemiluminescence have the requisite low limits of detection for hair analysis.
Radioimmunoassay. RIA has been used extensively both for screening and quantitating cocaine in hair. However, it was not appreciated by many of the earlier investigators that cocaine is the primary analyte in hair; therefore, some investigators used RIAs that were highly specific for BZE, whereas others used RlAs specific for cocaine. More recently, investigators have made their own modifications to commercially available reagent kits in an attempt to make their determinations more precise.
One of the earliest methods reported was a proprietary immunoassay procedure developed by Baumgartner and associates called RIAH (radioimmunoassay for hair). Unfortunately, the details of their proprietary methodology were not provided in any of their publications, which has prompted some editorial discussion in the literature (Sauls 1990; Needleman. 1991).
Graham and Koren have used RIA reagents from two manufacturers for both screening and quantitating cocaine and BZE in hair (Graham et al. 1989; Koren et al. 1992a, 1992b) For the analysis of cocaine they used a reagent kit (Coat-A-Count, Diagnostic Products, Los Angeles, CA) with antibodies that have a much higher affinity for cocaine than for BZE (cross-reactivity with BZE is 0.5 percent). They further modified the manufacturer's procedure by using cocaine hydrochloride standards (1 to 500 ng/mL) instead of the BZE standards provided. They reported a sensitivity for the assay of 0.025 ng cocaine/mg hair. For the analysis of BZE, they used a reagent kit containing antisera directed at the cocaine metabolite; however, there was a cross-reactivity with cocaine of 4 percent (Roche Abuscreen, Hoffman-La Roche). They reported a sensitivity of 0.25 ng BZE/mg hair.
Martinez and colleagues (1993) used the Coat-A-Count RIA kit with the BZE calibrators provided by the manufacturer. Hair extracts were centrifuged to separate particulate matter and then assayed directly. Their own cutoff concentrations were established by adding known amounts of BZE to 100-mg samples of drug-free hair and performing serial dilutions to give a final concentration in the ng/mL range. Their reported cutoff was 0.25 ng/mg hair expressed as combined cocaine and BZE, rather than BZE alone.
Fluorescence Polarization Immunoassay. Kintz and colleagues (1992) described the use of fluorescence polarization immunoassay (Abbott ADx) in screening hair samples for a variety of drugs including cocaine Their alkaline hydrolysates of hair samples were neutralized with HCI, half diluted with ADx buffer, and then analyzed directly for a positive or negative response according to the manufacturer's recommendations. They used the manufacturer's recommended cutoffs for plasma or urine, finding the method to be efficient, and because the antibody used can act directly on the hair hydrolysate, no prior purification was required.
Gas Chromatography/Mass Spectrometry. A variety of GC/MS methods have been reported for confirming and quantitating cocaine and its metabolites in hair. The methods differ with regard to the reagents used to derivatize BZE and EME, the type of mass spectrometer used (mass selective detector [MSD], ion trap, tandem mass spectrometer). or the ionization mode (electron impact or chemical ionization).
The authors' GC/MS procedure used ion-trap technology with chemical ionization and was developed specifically to quantitate the low levels of cocaine and metabolites likely to be found in hair following a single dose (Harkey et al. 1991). Hair digests were purified by bonded phase extraction, derivatized by adding 10 µL N-methyl-N-(tert-butyldimethylsilyl)-trifluoroacetamide (MTBSTFA), and incubated at 40 °C for 10 minutes. Aliquots (1 µL) were injected into the gas chromatograph/mass spectrometer for quantitation. Analysis was performed on a Finnigan model ITS-40 ion-trap mass spectrometer (San Jose, CA) equipped with a 15 m x 0.25 cm, 0.1 µm film thickness, DB-5 capillary column. The instrument was operated under chemical ionization conditions using isobutane as a reagent gas. Difluorococaine was used as an internal standard, rather than deuterated cocaine, because the studies required quantitation of both cocaine and pentadeuterated cocaine (d5-cocaine). The limit of quantitation for this method was 100 pg/mg hair for cocaine and BZE and 500 pg/mg for EME. The coefficient of variation was approximately 15 percent for cocaine and 25 percent for BZE and EME at concentrations less than 1 ng/mg. Chemical ionization was particularly useful for quantitation when the concentrations of cocaine and metabolites were low. Welch and colleagues (1993) also found methane chemical ionization particularly useful in quantitating cocaethylene in hair. The abundant molecular ion at 318 m/z (mass-to-charge ratio) and the base peak at 196 m/z are essentially the only peaks in the spectra. Cone and coworkers (1991) derivatized their acid extracts of hair (after partial purification by solid-phase extraction) with N,O- bis(trimethylsilyl) trifluoroacetamide (BSTFA) and 1 percent trimethylchlorosilane; they then analyzed aliquots using a Hewlett Packard model 5890A gas chromatograph interfaced with a model 5970B MSD (Palo Alto, CA), which was fitted with a cross- linked fused-silica capillary column (0.20 mm x 12 m). They reported a limit of detection of 0 1 ng/mg for all analytes using a 50-mg hair sample.
Nakahara and colleagues (1992) used hexafluoroisopropanol (HFIP) to derivatize BZE and pentafluoropropionic anhydride (PFPA) to derivatize EME. Hair samples were digested with proteinase K, purified by solid-phase extraction, derivatized with HFIP and PFPA, and then injected into a Hewlett Packard model 5890A gas chromatograph (Palo Alto, CA) fitted with a capillary column (0.25 mm x 25 m, 100 percent dimethyl polysiloxane) coupled to a model 5970B MSD operated in the selected ion monitoring mode. They reported a limit of detection of 0.3 ng/mg hair for cocaine, BZE, and EME.
Möller and colleagues (1992) enzymatically digested hair samples and then used solid-phase extraction before derivatization with a mixture of PFPA and pentafluoropropanol. Quantitation was performed with a Hewlett Packard model 5890A gas chromatograph (Palo Alto, CA) fitted with a capillary column (cross-linked 5 percent phenyl methyl silicone, 0.33 µm film thickness, 0.20 mm ID x 12 m). The model 5971A MSD was operated in the electron impact mode, and the limit of detection (using 10 to 30 mg hair was 0.1 ng/mg for cocaine and BZE and1 ng/mg for EME.
Tandem Mass Spectrometry (MS/MS). The relatively high cost of purchasing and maintaining tandem mass spectrometers has limited their use to relatively few laboratories. MS/MS analysis for cocaine in hair has been conducted primarily by the U.S. Naval Research Laboratory (Kidwell 1993), the Federal Bureau of Investigation (Martz 1988; Martz et al. 1991), and the National Institute of Standards and Technology (Welch et al. 1993). With MS/MS, intact hair could be analyzed directly or, usually, an extracted residue of hair was analyzed. For the analysis of intact hair specimens, a few strands of hair were washed, dried, cut into small (0.5 cm) pieces, and then placed in a solid probe. Segmental analysis was performed using approximately five hair strands that had been cut into sequential 0.5-cm sections. The probe was inserted into the first MS stage and heated at a relatively low temperature (110 °C) to remove any moisture. Next, the probe was heated to vaporize volatile components in the sample. The volatile compounds were ionized and then transmitted out of the first mass spectrometer into the second mass spectrometer where they were detected and analyzed.
Tandem mass spectrometers are powerful instruments and can generate large amounts of mass spectral data about the specimen under analysis. On the other hand, they are more dependent on tuning conditions and type of hair than quadrupole or ion-trap mass spectrometers. Quantitation can be confounded by contaminants in hair that cause instrument detuning, and if the probe is heated too quickly, false positive signals can be produced even with control drug-free hair. If the probe is heated too slowly, a bimodal desorption profile (e.g., two peaks for cocaine) can be observed. This is thought to result from the volatilization of loosely bound cocaine (drug absorbed on the hair surface) followed by the volatilization of cocaine from the hair cortex (Kidwell 1993).
Thus, MS/MS analysis of hair, especially using thermal desorption, may be more sensitive to hair type and to instrument-tuning conditions than the more routinely used GC/MS. Welch and colleagues (1993) found that when intact hair samples were analyzed, it became increasingly difficult to distinguish between the hair of drug users and blank hair as the number of samples increased, probably because of contamination from the hair or various pyrolysis products. They achieved better results when cryogenically powdered hair samples rather than short hair segments were used. All laboratories reported much better results using extracts from hair rather than direct analysis of hair. Thus, MS/MS may provide greater sensitivity than GC/MS for the quantitation of cocaine, BZE, and EME in hair; however, MS/MS's high cost makes GC/MS the preferred method for most laboratories.
The range of cocaine, BZE, and EME concentrations found in human hair obtained from a variety of sources is summarized in table 1. Cocaine concentrations were low and typically ranged from 0.1 (the detection limit reported by most investigators) to approximately 50 ng/mg. Concentrations of BZE were invariably lower (0.2 to 6 ng/mg), and the concentrations of EME were even lower (trace to 4.4 ng/mg). The ratio of cocaine to BZE and EME was highly variable (e.g., 1.3:10 for C:BZE and 1.2:22 for C:EME). Table 2 shows representative values from human subjects and from rats.
The concentration of these analytes in the hair of experimental animals after dosing with cocaine was also low (table 3), and the ratio of cocaine to its metabolites generally paralleled that observed in humans. The drug concentration in hair of the various test groups is discussed below.
TABLE 1. Range of cocaine, BZE, EME, and cocaethylene concentrations found in human hair
| Group | Self- Reported Dose |
Number of Subjects | Cocaine (ng/mg) | BZE (ng/mg) | EME (ng/mg) | CE (ng/mg) | Reference |
|---|---|---|---|---|---|---|---|
| Drug treatment | Heavy user | 10 | 6.4-19.2 | 0.3-2.5 | 0-1.9 | 0-2.6 | Cone et al. 1991 |
| Drug treatment | 0.04-5 g/month | 13 | -- | 0.007-6.4 | -- | -- | Baumgartner and Hill 1982 |
| Drug treatment | Unknown | 7 | 0.6-6.4 | -- | -- | -- | Balabanova and Hill 1982 |
| Drug treatment | 1-3 time/week | 6 | 0-5.7 | 0-1.1 | -- | -- | Harkey et al. 1991 |
| Coca chewers | 100 mg/day | 5 | 1.0-28.9 | 0.3-4.4 | 0-4.4 | -- | Henderson et al. 1992 |
| Coca chewers | 100 mg/day | 20 | 1.4-50.6 | 0.4-17.6 | Trace | -- | Möller et al. 1992 |
| Controlled- dose study |
0.6-4.2 mg/kg single dose |
25 | 0.1-5 | 0.1-0.36 | Trace | -- | Henderson et al. 1993 |
| Arrestees | Unknown | 22 | -- | 0.26-18 | -- | -- | Reuschel and Smith 1991 |
| Mothers | Occasional | 3 | 0.03-1.2 | -- | -- | -- | Graham et al. 1989 |
| Mothers | Frequent | 13 | 0.6-29.1 | -- | -- | -- | Graham et al. 1989 |
| Neonates | Unknown | 7 | 0.2-27.5 | -- | -- | -- | Graham et al. 1989 |
| Infants | Unknown | 2 | 4.3-7.8 | -- | -- | -- | Graham et al. 1989 |
KEY: BZE=benzoylecgonine; EME=ecgonine methyl ester; CE=cocaethylene
TABLE 2. Ratios of cocaine to metabolite concentrations reported in hair*
| Group | Doseż | Number | Cocaine:BZE | Cocaine:EME | Reference |
|---|---|---|---|---|---|
| Coca chewers | About 100 mg/day | 20 | 1.3-4.7 | 1.2-10.3 | Möller et al. 1992 |
| Drug treatment | Heavy users | 10 | 5-10 | 5.2-22 | Cone et al. 1991 |
| Coca chewers | About 100 mg/day | 5 | 2.1-8.6 | 6.6-15.5 | Henderson et al. 1992 |
| Controlled- dose study |
0.6-4.2 mg/kg single dose |
25 | 0.1-5 | -- | Henderson et al. 1993 |
| Rats | 5 mg/kg/day for 5 days |
7 | 10„ | 20.5 | Nakahara et al. 1992 |
*Drug and metabolite concentrations were determined by gas
chromatography/mass
spectrometry.
żThe amount of cocaine consumed was
estimated or self-reported, except for the
study involving the experimental
animals (rats).
„Mean value for seven animals.
KEY: BZE=benzoylecgonine;
EME=ecgonine methyl ester; CE=cocaethylene
Hair samples weighing 5.3 to 61.2 mg were collected from a group of 48 jail detainees, extracted with ethanol, and analyzed by RIA specific for BZE. Of these samples, 22 were positive, and GC/MS analysis showed that BZE concentrations ranged from 0.26 to 18 ng/mg hair (Reuschel and Smith 1991). The samples were collected anonymously, and there was no attempt to elicit a drug use history.
Baumgartner and colleagues (1982) used an RIA kit targeted at BZE to analyze hair samples from 13 admitted cocaine users in a drug rehabilitation program. Their self- reported cocaine use ranged from 0.04 to 5 g cocaine/month, and the drug concentrations found in their hair (reported as BZE equivalents) ranged from 0.007 to 6.4 ng/mg.
Cone and colleagues (1991) obtained hair specimens from 10 individuals who had completed a 180-day outpatient drug treatment program. The subjects identified themselves as heavy cocaine users; half the group identified themselves as intravenous users. The ranges of cocaine, BZE, and EME concentrations in their hair (quantitated by GC/MS) were 6.4 to 19.2 ng/mg, 0.3 to 2.5 ng/mg, and trace to 2.9 ng/mg, respectively. In addition, cocaethylene and norcocaine (chemical formulas shown in figure 1) were detected in low but quantifiable amounts in the hair of approximately half the subjects. The ratios of cocaine to metabolites varied among subjects and ranged from 5 to 10 for cocaine to BZE and from 5.2 to 22 for cocaine to EME. Balabanova and Homoki (1987), using an RIA for BZE, measured drug concentrations in the hair of seven cocaine users and their results were reported as the sum of cocaine and BZE. They reported drug concentrations in hair that ranged from 0.6 to 6.4 ng/mg. When these subjects were tested again 3 months later, the drug concentrations in hair had decreased to between 0.3 and 0.5 ng/mg.
The authors analyzed the hair of five subjects who had applied for participation in the clinical studies. These subjects were self-identified as experienced cocaine users, but none exhibited clinical signs of cocaine dependency. The concentration of cocaine in the hair of these subjects (quantitated by GC/MS) ranged from 0 to 5.7 ng/mg hair. There was little correlation between the concentration of cocaine in hair and self-reported drug use. For example, the highest concentration of cocaine was found in a moderate user (i.e., self-reported drug use was 1 to 2 times a week), whereas no cocaine was detected in the hair of a subject who reported heavy (i.e., daily) use of cocaine (detection limit 0.1 hair). BZE was found in the hair of only one subject and at a concentration of 0.1 ng/mg.
There has been increasing interest in the use of hair samples from pregnant women, neonates, and infants as a method for measuring in utero exposure to cocaine. Graham and colleagues (1989) used RIA to measure the concentrations of BZE in the hair of pregnant women. and neonates. The range of concentrations in those self-reporting as occasional users was 0.03 to 1.2 ng/mg and was 0.6 to 29.1 ng/mg in the hair of frequent users. Hair from seven neonates with a confirmed history of cocaine exposure had an average of 5.4 ng/mg BZE (range 0.2 to 27.5), whereas the hair of two infants (ages 2.5 and 3.5 months) had values of 4.3 and 7.8 ng/mg, respectively.
Hair obtained from South American native peoples provided interesting specimens for cocaine analysis. The native people of South America have chewed the leaves of the coca shrub for more than 3,000 years. Once prevalent throughout the continent, the custom today is restricted to Indians of Peru, Bolivia, Columbia, western Brazil, and northern Argentina (Carroll 1977). The concentration of cocaine in the plant material is variable, and there is also considerable variation in the use of coca; however, if one assumes a typical quid (a cut or wad) of coca leaves (10 to 20 g) contains approximately 0.5 percent cocaine, dry weight, then a conservative estimate of the daily dose of cocaine ingested by coca chewers is 50 to 100 mg. Absorption of the drug likely takes place in the buccal cavity as well as in the intestine, and significant blood levels of cocaine (about 100 to 200 ng/mL at peak) following coca chewing have been reported (Holmstedt et al. 1979, Paly et al 19B0. pp. 86-89). This was a significant concentration but less than that reported for cocaine abusers (Jaffe 1989, pp. 535- 584). Therefore, these hair samples might have been representative of someone who ingested approximately one line (approximately 25 mg) of cocaine per day.
The authors analyzed the hair from five South American Indians who acknowledged chewing coca leaves daily. Cocaine concentration in the hair of these subjects ranged from 1.0 to 28.9 ng/mg; BZE ranged from 0.3 to 4.4 ng/mg; and EME ranged from 0.5 to 4.4 ng/mg. The finding that cocaine was present at approximately 5 times higher concentration than BZE and approximately 10 times higher than EME was surprising considering these individuals likely had high steady-state plasma concentrations of BZE. It was also interesting that washing the hair before analysis not only reduced the concentration of cocaine but also reduced the concentration of BZE and EME as well. This finding, which has been reported by others (Cone et al. 1991), suggests that the washing procedures typically used in hair analysis probably extract analytes from the hair cortex as well.
The authors performed a series of controlled-dose experiments in which isotopically labeled cocaine (benzoyl-d5-cocaine-HCI) was administered intrravenously or intranasally to 25 human volunteers under controlled clinical conditions (Henderson et al. 1993). Sequential blood and sweat were collected for up to 3 days, and hair samples were collected for up to 10 months. All samples were analyzed by chemical ionization GC/MS for cocaine-d5; and its metabolite benzoylecgonine-d5 (BZE-d5). The use of isotopically labeled cocaine distinguished between administered drug and any cocaine used by the subjects either before or during the study. In both hair and sweat, the predominant analyte was the parent drug cocaine-d5. In contrast, BZE-d5 was the major analyte in blood, especially after approximately 2 hours. The sevenfold range of cocaine doses used in the study (0.6 to 4.2 mg/kg) resulted in 0.1 to 5 ng of cocaine-d5 per hair sample and approximately one-sixth that amount of BZE-d5. The minimal detectable dose by this GC/MS procedure was estimated as approximately 25 to 35 mg drug administered intravenously, which is approximately the amount found in a single line of cocaine. The authors found a poor correlation between the dose of drug administered and the amount of cocaine-d5 incorporated into hair. Non-Caucasians in particular incorporated considerably more cocaine-d5 into hair than did Caucasians (from 2 to 12 times, depending on how it was measured). These interindividual differences could not be explained by differences in the individuals' plasma pharmacokinetics. Also, there was little correlation between the time of drug administration and the position of drug along the hair shaft. Segmental analysis of the hair samples revealed that some subjects who received only a single dose had cocaine-d5 distributed along most of the hair shaft, whereas some subjects who received multiple doses had the drug confined to a much smaller area. In addition, cocaine-d5 was detected in hair as early as 8 hours after drug administration. Hair was obtained from four subjects 1 and 3 days after they received 0.6 mg/kg of cocaine-d5 intranasally; cocaine-d5 was found in three of the four subjects, which suggests that sweat or sebum may play a role in the incorporation of some drugs into hair.
Cocaine and metabolites have been detected in the hair of laboratory animals following cocaine administration. The species studied to date include sheep (Balabanova et al. 1987), mice (Pirozhkov et al 1992; Poet et al 1992), guinea pigs (Koren et al. 1992b). and rats (Michalodimitrakis 1987). In general, the drug concentrations in their hair are similar to those found in human hair, and the ratios of cocaine to its metabolites appear to be similar as well. Nakahara and colleagues (1992) analyzed hair from rats that had received 5 mg/kg/day intraperitoneally (IP) for 5 days and found the average cocaine in hair to be 16.4±4.8 ng/mg; however, these researchers did not report the range of drug concentrations found. BZE and EME were present in approximately tenfold lower concentrations. Nakahara and colleagues (1992) also studied the plasma pharmacokinetics of cocaine in their test animals and found the amounts of cocaine and metabolites in hair did not correlate well with the concentrations of drugs in the hair. They suggested that drug incorporation into hair must depend on the physiochemical properties of the drug.
Balabanova and Homoki (1987) administered cocaine to sheep for 12 days (dose was not indicated) and found cocaine concentrations in wool in the range of 2 to 3 ng/mg (measured by RIA); these concentrations remained relatively constant for the next 60 days. Pirozhkov and coworkers (1992) administered 20 mg/kg cocaine, cocaethylene, or cocaine and ethanol twice daily to mice for 3 weeks and (measuring with GC) found cocaine concentrations in hair that ranged from 0.9 to 2.4 ng/mg and cocaethylene concentrations that ranged from 2 4 to 2.8 ng/mg. In the animals that received cocaine plus ethanol, cocaethylene could not be detected. Pirozhkov's group reasoned that hair from cocaine and alcohol abusers contains much less cocaethylene than cocaine; thus, the expected concentration of cocaethylene in the hair of the mice would be below the limit of detection for their method.
TABLE 3. Range of cocaine, BZE, EME, and cocaethylene concentrations found in animal hair
| Species | Dose | Number | Cocaine (ng/mg) |
BZE (ng/mg) |
EME (ng/mg) |
Reference |
|---|---|---|---|---|---|---|
| Rat | 5 mg/kg/day (IP) for 5 days |
9 | 16.4±4.8 | 1.7±0.4 | 0.8±0.3 | Nakahara et al. 1992 |
| Rat | 3 mg/kg/day (IM) for 10 days |
7 | -- | 0.02±0.05 | -- | Michalodimitrakis 1987 |
| Mouse | 40 mg/kg/day (IP) for 3 weeks |
26 | 0.8-2.4 | -- | -- | Pirozhkov et al. 1992 |
| Mouse | 20-50 mg/kg/day (IP) for 6 weeks |
20 | 316-1,609 | -- | -- | Poet et al. 1992 |
| Sheep | 2 mg/kg/day (IV) for 40 days |
Not specified | 1.5-3.0 | -- | -- | Balabanova and Homoki 1987 |
KEY: BZE=benzoylecgonine; EME=ecgonine methyl ester; IP=intraperitoneally;
IM=intramuscular; IV=intravenously
In another study using mice as experimental animals, Poet and colleagues (1992) administered cocaine IP at 20 mg/kg/day for the first week, 30 mg/kg/day for the second week, and 50 mg/kg/day for the third week. They reported extremely high concentrations of cocaine in the hair of the mice. Hair samples were extracted with HCl, and cocaine concentrations (determined by an RIA specific for cocaine) ranged from 316 to 1,609 ng/mg. These concentrations are many orders of magnitude higher than have been reported in human or other animal hair and are difficult to explain. There was a significant variation in the amount of drug incorporated into hair of similarly treated and genetically identical animals.
Michalodimitrakis (1987) injected rats daily with 3 mg cocaine administered intramuscularly for 10 days and analyzed hair samples for BZE using RIA. No drug could be detected for the first 4 days; thereafter, BZE concentrations in hair ranged from 0.024 to 0.048 ng/mg.
No single method has emerged as the best or only procedure; however, RIA and GC/MS are the most commonly used screening and confirmation techniques. Because of RIA's speed, sensitivity, and relatively low cost, most laboratories use this screening method. However, because cocaine is the primary analyte in hair, the antisera should be directed at cocaine rather than BZE, as is the case with many RIA kits used in urine drug screening. Analysts also should be aware that most commercially available RIA kits are designed for urine specimens and therefore have not been evaluated for possible matrix effects from different hair digestion techniques or for cross-reactivity to other possible components in hair, such as cosmetics.
Capillary column GC/MS using deuterated internal standards is becoming the preferred method for confirming and quantitating cocaine in hair. A variety of reagents have been used for derivatizing BZE and EME, including BSTFA, HFIP, MTBSTFA, and PFPA. No reagent appears superior, and the choice probably is determined by the laboratories' existing equipment and their prior experience with the reagent. The electron impact mode is more commonly employed (probably because it is more prevalent); however, chemical ionization may be more useful to measure analytes such as norcocaine and cocaethylene that are present in low levels.
MS/MS can be a powerful technique when great sensitivity is required and can work well using extracts of hair. However, when thermal desorption is used, its performance is more easily affected by hair type, sample size, tuning conditions, and the rate at which the solid probe is heated.
The correlation between the dose of cocaine and the amounts of drug and metabolites detected in hair is unclear at the present time and remains controversial. Most studies have shown few correlations between dose of drug and concentration found in hair.
Self-Reported Drug Use. Data from studies comparing hair analysis results with self-reported drug use are conflicting; however this might be expected because in many cases the minor metabolite BZE is quantitated, and it is fairly well accepted that self-reports drug use may be inaccurate. Subjects may deliberately mislead, forget, or simply be unaware of the amount or purity of drug use.
Baumgartner and colleagues (1982) reported that cocaine concentrations in hair correlated approximately with the severity of drug use. Similarly, Graham and coworkers (1989) found that BZE levels in the hair of 3 women in their study who self-identified as occasional users were significantly lower than the BZE concentrations of the 13 women who self-identified as frequent users.
On the other hand, Martinez and colleagues (1993) analyzed hair samples from Hispanic males attending a community outreach program and concluded that hair analysis could identify drug use that was not detected by urinalysis; however, there was no particular relationship between the stated frequency of cocaine use and the levels of cocaine and BZE in the hair of their subjects. Möller and colleagues (1992) found no correlation between concentrations of cocaine or metabolites in hair and the reported use patterns of South American coca chewers.
The authors sampled five subjects who were experienced cocaine users and found little correlation between their reported use and the cocaine concentration in their hair (Harkey et al. 1991). In fact, one subject said he used cocaine more than three times a week, yet there was no cocaine or metabolite detected in his hair.
Controlled-Dose Studies. The authors administered precise doses of cocaine-d5 to 25 subjects under controlled clinical conditions and used GC/MS to quantitate the cocaine, BZE, and EME incorporated into hair (Henderson et al. 1993). A poor correlation was found between the dose administered and the amount of drug (cocaine-d5) incorporated into hair. Under certain circumstances, increased doses did result in an increased amount of drug incorporated into hair; however, because of the considerable intersubject variability, it was impossible to infer the dose administered from the amount of drug in hair. However, the doses administered in the study were limited because of ethical and safety considerations and were no doubt considerably less than what might be used by compulsive cocaine users.
The authors found that it was difficult to estimate precisely the time of drug administration from the position of cocaine along the hair shaft. In some subjects' hair, sectional analysis showed that cocaine was confined to a relatively small segment of the hair shaft and that it migrated with time along the axis at a rate of about 1 cm/month, the reported hair growth rate for humans (Saitoh et al. 1969, pp. 183-201; Montagna and Parakkal 1974, pp. 83-105). However, in many subjects little correlation was found between the position of the drug along the shaft and the time since drug administration. Variables that contribute to inaccuracies in correlating position of drug along the hair shaft with the time of drug ingestion include intersubject differences in hair growth rate, measuring hairs in different phases of their growth cycle, and variability in alignment of the hair strands prior to cutting. Hair growth rate can vary threefold among individuals (0.5 to 1.5 cm/month) (Saitoh et al. 1969; Montagna and Parakkal 1974, pp. 83-105).
Uematsu and colleagues (1993) used a single dose of a quinolone antimicrobial to measure hair growth rate in human volunteers and found the drug was distributed widely along the hair shafts (5 to 7 cm) when five strands of hair were measured. However, when only one strand of hair was measured, the drug was restricted to a 1- to 2-cm portion of the shaft. This suggests that there may have been some variability in aligning the hair prior to cutting or that not all hairs in the sample were in the growing phase when the drug was ingested. Finally, because cocaine is excreted in sweat, it is possible that the drug may be incorporated directly into hair as the hair emerges from the scalp.
The possibility that a positive hair test for cocaine could result from external contamination is an important consideration when interpreting the results of a hair test. A few investigators have shown that washing can remove all externally deposited cocaine (Baumgartner and Hill 1992, pp. 577-597; Koren et al. 1992b). Others have found that hair exposed to cocaine in aqueous or other solution will accumulate large amounts of the drug; that washing will remove most, but not all, of the accumulated drug; and that the amount remaining will be consistent with very heavy drug use (Cone et al. 1991; Henderson et al. 1991; Welch et al. 1993; Blank and Kidwell 1993). The National Institute of Standards and Technology prepares reference materials by soaking hair in a solution of dimethylsulfoxide containing cocaine, BZE, morphine, and codeine. These materials are provided to laboratories as proficiency samples to evaluate the accuracy and precision of the laboratories' methods. Until this issue is resolved, external contamination should always be considered when interpreting hair analysis data, and both the collection site and the testing laboratory should have rigorous quality control measures to prevent contamination of any specimen. It has been proposed that unique metabolites such as cocaethylene or norcocaine be used as indicators of active exposure; that is, these metabolites, as well as cocaine and BZE, should be present in any hair sample declared positive (Cone et al. 1991). However, these metabolites are present in low, often undetectable, concentrations. In addition, cocaethylene or norcocaine has been identified in contraband cocaine samples, although this is likely a rare occurrence (Janzen 1992).
The possibility that drug incorporation into hair differs with hair type has been raised by several investigators and remains controversial. Kidwell (1993) found that thick, black hair takes drugs more slowly from solution and releases them more slowly than fine, brown hair. Uematsu and coworkers (1993) have suggested that certain drugs bind to melanin and found that in Japanese subjects the concentrations of haloperidol, chlorpromazine, nicotine, and quinolone antimicrobials are higher in black hairs than in the "grizzled" (i.e., white) hairs of their subjects. In the authors' controlled-dose study with cocaine-d5, all non-Caucasians (i.e., African- Americans, Hispanics, and East Indians) in the study incorporated significantly higher levels of cocaine-d5 into their hair (Henderson et al. 1993). The plasma pharmacokinetics for cocaine in these subjects were not significantly different from the Caucasian subjects.
Over the past decade, several methods for analyzing hair for cocaine have been developed, and laboratories throughout the world are conducting such tests. No single method or combination of methods has been accepted as a benchmark or standard. Because so many methods have been used, some specific and some nonspecific, it is difficult to compare many of the data, and serious questions still remain about how to interpret the results of a hair test for cocaine.
Nevertheless, the following is known about cocaine hair analysis: In humans (and experimental animals), cocaine ingestion can be detected by analyzing hair (or fur). Even a single dose of cocaine can be detected if sensitive methods are employed. Increasing doses usually result in increased levels of the drug and metabolites in hair; however, a clear dose- response relationship has not been established. This is not unexpected given the variety of methods that have been used and the paucity of controlled dose studies. Also, it may be that the physicochemical characteristics of cocaine-for example, its high lipid solubility- allow this drug access to hair through alternate routes such as sweat and sebum. At the very least, it is known that the pharmacokinetics of cocaine incorporation into hair do not mirror its pharmacokinetics in plasma because cocaine is the major analyte in hair and the metabolites BZE and EME are present in low and variable amounts in hair.
Hair sampling procedures are becoming more standardized. Samples to be tested for cocaine are most often obtained from the scalp, usually from the posterior vertex region that has a high population of hair follicles in the growing stage. However, some researchers have advocated using axillary or pubic hair because hair from these regions would less likely be contaminated from an external cocaine source. Unfortunately, little is known about the relative rates of cocaine incorporation into various types of hair or what role sweat may play. Even when a common sampling site is used, such as the posterior vertex region, there are indications that different hair types may preferentially incorporate cocaine. This so called "racial bias" has not been established, but it should receive more attention.
Finally, the effectiveness of washing procedures in removing cocaine absorbed or incorporated from the external environment remains controversial. This has important implications for the forensic application of cocaine hair testing results. At the very least, the lack of standardized washing procedures continues to make interlaboratory comparisons of data problematic.
In conclusion, there are several sensitive methods now available for the analysis of cocaine and metabolites in hair. However, until the testing technology is standardized, the mechanisms of cocaine incorporation into hair better understood, and unequivocal procedures for distinguishing between drug ingestion and external contamination developed, the results from hair tests for cocaine should be interpreted with caution.
Arnold. W., and Püschel, K. Experimental studies on hair as an Indicator of past or present drug use. J Forensic Sci Soc 21:83, 1981.
Balabanova, S., Brunner, H., and Nowak, R. Radioimmunological determination of cocaine in human hair. Z Rechtsmed 98:229-234, 1987.
Balabanova, S., and Homoki, J. Determination of cocaine in human hair by gas chromatography/mass spectrometry Z Rechtsmed 98:235-240, 1987.
Balabanova, S., and Wolf, H.U. Methadone concentrations in human hair of the head, axillary and pubic hair. Z Rechtsmed 102(5):293-296, 1989.
Baumgartner, W. Hair analysis for drugs of abuse. Biweekly Reporter 3:442-446, 1989.
Baumgartner. W.A., Black, C.T., Jones, P.F.; and Blahd, W.H. Radioimmunoassay of cocaine in hair: Concise communication. J Nucl Med 23(9):790-792, 1982.
Baumgartner, W.A., and Hill, V.A. Hair analysis for drugs of abuse: Decontamination issues. In: Sunshine, I., ed. Recent Developments in Therapeutic Drug Monitoring and Clinical Toxicology. New York: Marcel Dekker, 1992.
Blank, D.L., and Kidwell, D.A. External contamination of hair by cocaine: An issue in forensic interpretation. Forensic Sci Int 63(1-3):145-156, 1993.
Carroll, E. Coca: The plant and its use. In: Petersen, R.C., and Stillman, R.C., eds. Cocaine: 1977. National Institute on Drug Abuse Research Monograph 13. DHHS No. (ADM) 82471. Washington, DC: Supt. of Docs., U.S. Govt. Print. Off., 1977.
Chatt, A., and Katz, S.A. The biological basis for trace metals in hair. In: Katz, S.A., and Chatt, A., eds. Hair Analysis: Applications in the Biomedical and Environmental Sciences. New York: VCH, l988.
Cone, E.J., Yousefnejad, D., Darwin, W.D, and Macquire, T. Testing human hair for drugs of abuse. II. Identification of unique cocaine metabolites in hair of drug abusers and evaluation of decontamination procedures . J Anal Toxicol 15(5):250-255, 1991.
Gill, P., Jefferies, A.J., and Werrett, D.J. Forensic applications of DNA fingerprints. Nature 318:577-579, 1985.
Graham, K., Koren, G., Klein, J., Schneiderman, J., and Greenwald, M. Determination of gestational cocaine exposure by hair analysis. JAMA 262:3328-3330, 1989.
Harkey, M.R., Henderson, G.L., and Zhou, C. Simultaneous quantitation of cocaine and its major metabolites in human hair by gas chromatography/chemical ionization mass spectrometry. J Anal Toxicol 15(5):260-265, 1991.
Henderson, G.L., Harkey, M.R., and Jones, R.T. "Hair Analysis for Drugs of Abuse." Contract No. NIJ 90-NIJ-CX-0012. Final report submitted to the National Institute on Drug Abuse and National Institute of Justice. September 1993.
Henderson, G.L., Harkey, M.R., Jones, R.T., and Zhou, C. "Effect of External Contamination on the Analysis of Hair for Cocaine." Paper presented at the Joint Meeting of Forensic Toxicologists and the Canadian Society of Forensic Scientists, Montreal, Quebec, Canada, September 23-27, 1991.
Henderson, G.L., Harkey, M.R., Zhou, C., and Jones, R.T. Cocaine and metabolite concentrations in the hair of South American coca chewers. J Anal Toxicol 16(3):199-201, 1992.
Holmstedt, B., Lindgren, J., Rivier, L., and Plowman, T. Cocaine in blood of coca chewers. J Ethnopharmacol 1 :69-78, 1979.
Jaffe, J.H. Drug addiction and drug abuse. In: Goodman, A.; Goodman, L.S.; and Gilman, A., eds. The Pharmacological Basis for Therapeutics. New York: Macmillan, 1989.
Janzen, K. Concerning norcocaine, ethylbenzoylecgonine, and the identification of cocaine use in human hair. (Letter.) J Anal Toxicol 16(6):402, 1992.
Jatlow, P. Cocaine by GLC and a nitrogen detector. In: Sunshine, 1., ed. Methodology of Analytical Toxicology. Boca Raton, FL: CRC Press, 1975.
Kidwell, D.A. Analysis of phencyclidine and cocaine in human hair by tandem mass spectrometry. J Forensic Sci 38(2):272-284, 1993.
Kintz, P., Kieffer, I., Messer, J., and Mangin, P. Nicotine analysis in neonates' hair for measuring gestational exposure to tobacco. J Forensic Sci 38:119-123, 1993.
Kintz, P., Ludes, B., and Mangin, P. Detection of drugs in human hair using Abbott ADx with confimnation by gas chromatography/mass spectrometry (GC/MS). J Forensic 37:328-331, 1992.
Koren, G., Klein, J., Forman, R., and Graham, K. Hair analysis of cocaine: Differentiation between systemic exposure and external contamination. J Clin Pharmacol 32(7):671-675, 1992a .
Koren. G., Klein, J., Forman, R., Graham, K., and Phan, M.K. Biological markers of intrauterine exposure to cocaine and cigarette smoking. Dev Pharmacol Ther 18:228-236, 1992b.
Martinez, F., Poet, T.S., Pillai, R., Erickson, J., Estrada, A.L., and Watson, R.R. Cocaine metabolite (benzoylecgonine) in hair and urine of drug users. J Anal Toxicol 17(3):138-142, 1993.
Martz, R., Donnelly, B., Fetterolf, D., Lassewell, L., Hime, G.W., and Hearn, W.L. The use of hair analysis to document a cocaine overdose following a sustained survival period before death. J Anal Toxicol 15(5):279- 281, 1991.
Martz, R.M. The identification of cocaine in hair by GC/MS and MS/MS. Crime Lab Digest 15(3):67-73, 1988.
Michalodimitrakis, M. Detection of cocaine in rats from analysis of hair. Med Sci Law 27(1): 13-15, 1987.
Möller, M.R., Fey, P., and Rimbach, S. Identification and quantitation of cocaine and its metabolites, benzoylecgonine and ecgonine methyl ester, in hair of Bolivian coca chewers by gas chromatography/mass spectrometry. J Anal Toxicol 16(5):291-296, 1992.
Montagna, W., and Parakkal, T.F. The Structure and Function of Skin. 3rd ed. New York: Academic Press, 1974.
Nakahara, Y., Ochiai, T., and Kikura, R. Hair analysis for drugs of abuse. V. The facility in incorporation of cocaine into hair over its major metabolites, benzoylecgonine and ecgonine methyl ester. Arch Toxicol 66:446449, 1992.
Needleman, S.B. Further discussion on discussion of hair analysis of abuse. (Letter.) J Forensic .Sci 36(3):629-630, 1991.
Paly, D., Jatlow, P., Van Dyke, C., Balieses, F., and Byck, R. Plasma levels of cocaine in native Peruvian coca chewers. In: Jeri, F.R., ed. Interamerican Seminar on Medical and Sociological Aspects of Coca and Cocaine. Lima, Peru: Pan American Health Office, World Health Organization, 1980.
Pirozhkov, S.V., Watson, R.R., and Eskelson. C.D.. Gas chromatographic detection of cocaine and cocaethylene in hair of mice chronically injected with cocaine or cocaethylene and fed ethanol. Forensic Sci Int 57:99-107, 1992.
Poet, T., Martinez, F., and Watson, R.R. Effects of murine retroviral infection on hair and serum levels of cocaine and morphine. Forensic Sci Int 54:29-38, 1992.
Reuschel, S.A., and Smith, F.S. Benzoylecgonine (cocaine metabolite) detection in hair samples of jail detainees using radioimmunoassay (RIA) and gas chromatography/mass spectrometry (GC/MS). J Forensic Sci 36(4):1179- 1185, 1991.
Saitoh, M., Usuka, M.; Sakamoto, M. and Korobi, T. Rate of hair growth. In: Montagna, W., and Dobson, R.L., eds. Advances in the Biology of Skin. Hair Growth. Oxford: Pergamon, 1969.
Sauls, F.C. Discussion of hair analysis for drugs of abuse. (Letter.) J Forensic Sci 35:778, 1990.
Smith, F.P., and Liu, R.H. Detection of cocaine metabolite in perspiration stain, menstrual bloodstain, and hair. J Forensic Sci 3: 1269-1273, 1986.
Uematsu, T., Nakano, M., Akiyama, H., and Nakashima, M. The measurement of a new antimicrobial quinolone in hair as an index of drug exposure Br J Clin Pharmacol 35(2):199-203, 1993.
Valente, D., Cassini, M., Pigliapochi, M., and Vansetti, G. Hair as the sample in assessing morphine and cocaine addiction. Clin Chem 27(11): 1952-1953, 1981.
Welch, M.J., Sniegoski, L.T., Allgood, C.C., and Habram, M. Hair analysis for drugs of abuse: Evaluation of analytical methods, environmental issues, and development of reference materials. J Anal Toxicol 17(7):389-398, 1993.
This chapter was prepared with support from National Institute on Drug Abuse grant RO1-DA082228, "Hair Analysis for Drugs of Abuse."