

|
| [3D .mol Image] |
Then comes the name of the compound itself containing, as a rule, either three of four letters. The first letter either tells the number of the groups present, or it can be the first of these groups. As to number:
1
2
3
4
5
6a
b
g
d
o
N
O
S
As to group names:
N is for nitrogen to which a single group is attached - indicates mono-substitution D is for di-substitution T is for tri-substitution
And the last letter defines the class:
aliphatic alkyl groups groups with hetero-atoms M is for methyl
E is for ethyl
P is for propyl
IP is for isopropyl
B is for butyl
IB is for isobutyl
SB is for sec-butyl
TB is for tert-butyl
A is for amyl
AL is for allyl
H is for hexylHO is for hydroxy
MeO is for methoxy
MeS is for methylthio
MDO is for methylenedioxy
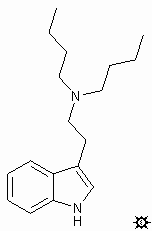
In this way, a monster such as 5,a,N-TTBT becomes, quite obviously, a tryptamine with three tert-butyl groups attached, one at the 5-position, one at the alpha-carbon next to the amine, and one on the amine nitrogen atom itself.
T is for tryptamine
C is for carboline
S is for serotonin
| HTML and Design by Bo & Erowid | Used by Erowid with permission of author |