[3D .mol structure]


|
|
[3D .jpg image] [3D .mol structure] |
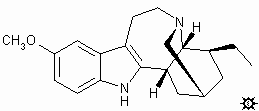
Tabernanthe iboga. This is the major source of ibogaine and is found in Gabon, mentioned above.And there are many plants in the Apocynaceae family that carry fascinating alkaloids that are closely related in structure to ibogaine and which, potentially, might have a similar psychopharmacology. In most of these, ibogaine is present in very small amounts, if any at all.
Tabernanthe orientalis. This plant is now called Ervatamia orientalis, and is found in Western Australia. The leaves contain ibogaine, along with six minor alkaloids that are closely related, structurally.
Tabernanthe pubescens. This is found in Zaire, and contains a number of alkaloids closely related to ibogaine in structure, as well as ibogaine itself.
Tabernaemontana spp. This genus is from a tribe within the family Apocynaceae that is called the Tabernaemontaneae. As an official sub-family it would be called Tabernaemontanoideae. It is because of the casual use of names such as these that botanical binomialists are rarely invited to social functions. It (this Genus, that is) contains several dozen species, some with ibogaine, many with analgesic or sedative action in experimental animals, and some with a quite a history of native usage either in Africa or Southeast Asia.
Anacampta spp. have usually been published as Tabernaemontana spp., as have been species originally published as part of the Genera Bonafousia, Capuronetta (which has become the species capuronni under this Genus), Conopharyngia, Ervatamia, Gabunia, Hazunta, Muntafara, Pagiantha, Pandaca, Peschiera, Phrissocarpus, and Stenosolen, All of these contain alkaloids related to Ibogaine.
Callichilia barteri has appeared as Hedranthera barteri, but C. subsessilis demands the name Tabernaemontana subsessilis in the presentation of its alkaloid content.
Creoceras, Rejoua, Schzozygia, Stemmadenia and Voacanga, have, with all their species, remained intact with their original names.
Peschiera echinata. This is one of some ten species within the Tabernaemontaneae classification, with some 2% alkaloid content in its leaves. Ibogaine is present.
Voacanga schweinfurthii var. puberula (known in the older literature as Voacanga puberula) contains some ten related alkaloids, the major one of which is found in the seeds, and is tabersonine present at a rather remarkable 3.5 %. Ibogaine is present in the root bark but, at a concentration of 200 mg/Kg (0.02%), it is truly a minor constituent.
| [ |
[Main Index] | [Forward |
| HTML and Design by Bo & Erowid | Used by Erowid with permission of author |
|
[Plants & Drugs]
[Mind & Spirit]
[Freedom & Law]
[Arts & Sciences]
[Library]
[Search]
[About] (html and design © 1995-2009 Erowid.org. Please ask permission before publicly reproducing.) (Contents © respective copyright holders.) |
|---|