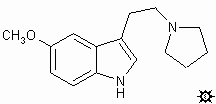


|
| [3D .mol structure] |
| [ |
[Main Index] | [Forward |
| HTML and Design by Bo & Erowid | Used by Erowid with permission of author |
|
[Plants & Drugs]
[Mind & Spirit]
[Freedom & Law]
[Arts & Sciences]
[Library]
[Search]
[About] (html and design © 1995-2009 Erowid.org. Please ask permission before publicly reproducing.) (Contents © respective copyright holders.) |
|---|