PHYTOCHEMICAL
ANALYSIS Phytochem. Anal. 10, 22–25,
(1999) High Performance Liquid
Chromatographic Quantification of Salvinorin
A from Tissues of Salvia divinorum Epling & Játiva-M John
W. Gruber, Daniel J. Siebert, Ara H. Der Marderosian and Rick S. Hock A reversed‑phase high
performance liquid chromatographic method for the determination of salvinorin
A, a psychotropic diterpene isolated from the Mexican sage Salvia divinorum, has been developed. Extracts from several plant collections were
examined on a C‑18 column with UV detection and isocratic elution with
acetonitrile: water (45:55). This assay allowed quantification of salvinorin A
in extracts of leaves and stems of S. divinorum and has also
been applied to the screening of related species for the production of
salvinorin A. Levels of salvinorin A in leaves range from 0.89 to 3.70 mg/g dry
weight. © 1999 John Wiley & Sons, Ltd. Keywords: Hallucinogen; high performance liquid chromatography; Mexican sage;
psychotropic diterpene; Salvia divinorum; salvinorin A. INTRODUCTION Salvia divinorum Epling et Jdtiva‑M. (Lamiaceae) is
native to Oaxaca in Central Mexico. Among the indigenous Mazatec people of
Oaxaca, S. divinorum has long been
used in ceremonial healing rituals as a means of inducing a visionary state
that allows the participants to divine the cause of illness or ailment (Wasson,
1962; Valdés et al., 1983). The
psychotropic activity of this mint species has been attributed to a
neoclerodane diterpene found in the leaves, salvinorin A (1) (Ortega et al., 1982;
Valdés et al., 1984, 1987; Siebert,
1994). It has been demonstrated that salvinorin A is an extraordinarily potent
drug in humans, exhibiting threshold effects at doses in the range of 200‑500
micrograms, making it the most potent natural hallucinogen ever studied
(Siebert, 1994). The site of action and mechanism of pharmacological activity
of 1 remain unknown. When submitted for major neurotransmitter and
second messenger receptor site screening, 1 showed no significant
binding to these sites at concentrations of 10‑5 M (Siebert, 1994). It has been suggested that
the extraordinary potency of salvinorin A, and the absence of any known
mechanism for its effects, could indicate that a new receptor system is
involved in the activity of the compound (McKenna, 1996). 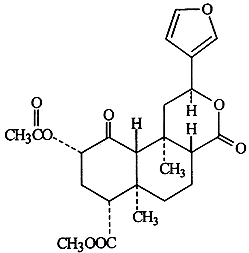 1 Salvinorin A has thus far been
attributed uniquely to S. divinorum; no
other natural source for this compound has been identified nor has it been
synthetically produced. The current distribution of S. divinorum suggests that all existing stands of the plant have been
intentionally cultivated by humans; no clearly wild populations of the species
have been identified (Reisfeld, 1993). Furthermore, while the plant is readily
propagated through asexual reproduction by rooting stem cuttings, it appears
that it rarely if ever reproduces from seed in nature. It has been proposed
that S. divinorum may in fact be a
hybrid, resulting in substantially reduced fertility within the species
(Reisfeld, 1993). The parentage for such a hybrid may remain unknown as no
obvious candidates have been proposed as likely progenitors. Since its initial isolation, no
analytical assay has been published in order to quantify precisely the amount
of salvinorin A present in samples of S. divinorum
or any other plant species.
The purpose of the current study was to develop a sensitive quantitative assay
to determine the amount of 1 present in leaf samples of S. divinorum. In addition, the method
has been used to screen for this compound in other species. EXPERIMENTAL General experimental procedures. Salvinorin A (1) used for standard curve development was purified from S. divinorum leaf extracts and
authenticated by nuclear magnetic resonance spectroscopy and by thin layer
chromatographic (TLC) comparison with authentic 1 (Valdés et al., 1994).
All solvents used for extraction and chromatography were of high performance
liquid chromatographic (HPLC) grade from Fisher Scientific (Fair Lawn, NJ,
USA). Water used in HPLC mobile phase mixtures was distilled and subsequently
filtered through a 0.22 ~im membrane (Millipore Corp., Bedford, MA, USA). Plant materials. Asexually propagated plants of S. divinorum used in establishing the HPLC
methods were purchased from Logee's Greenhouses (Danielson, CT, USA) and
multiplied by rooting additional cuttings in the S.B. Penick Experimental
Greenhouse at the Philadelphia College of Pharmacy and Science. Voucher
specimens were deposited in the herbarium of the Philadelphia College of
Pharmacy and Science. Additional S. divinorum
samples used for analysis were collected from established outdoor stands. Extraction of samples. Plant tissues were lyophilized after harvest
and ground to a homogeneous powder in a Wiley mill (no. 20 mesh). Samples
(0.500 g) of lyophilized whole leaf were extracted in 125 mL roundbottom flasks
by steeping in 25 mL of chloroform for 30 min. The extract was filtered and the
filtrate set aside. The extraction flask and filtered solids were rinsed with
an additional 15 mL of fresh chloroform. The filtrate from the rinse was then
combined with the original filtrate and the resulting solution was evaporated
to dryness with a rotary evaporator. The dry solids were redissolved in a
mixture of 20.0 mL methanol and 5.0 mL acetone using sonication to assist in
dissolving of all solid material. Chromatography conditions. HPLC was performed using a Milton Roy HPLC
system (Riviera, FL, USA), consisting of a Constametric 3000 Series isocratic
pump, a Rheodyne injector (Rheodyne L.P., Cotati, CA, USA), and a
Spectromonitor 3100 variable wavelength UV‑VIS detector. The analogue
detector output was acquired by an advanced computer interface (Dionex Corp.,
Sunnyvale, CA, USA), converted to a digital signal, and then processed by Al‑450
Chromatography Automation Software (Dionex). The HPLC column was a Zorbax
(MACMOD Analytical, Inc., Chadds Ford, PA, USA) 300 SBC18 column (250 x 4.6 mm
i.d.; 5 micrometer particle diameter; 300 A average pore size). In order to
protect the integrity of the analytical column, all analyses were performed
with a coupled C‑18 guard column. A mobile phase of acetonitrile:water
(45:55) was used for all analyses at a flow‑rate of 1.0 mL/min, and 1 was detected by UV absorption at 208
mn. Composite standard curve. A standard curve for 1 was produced
using a solution of 0.051 mg/mL of standard dissolved in the HPLC mobile phase.
By varying injection size, six different amounts of salvinorin A (0.255, 0.510,
0.765, 1.02, 1.28 and 1.53 [tg) were chromatographed: for each amount analyzed,
three injections were made. In order to
validate the HPLC method, three separate determinations of the standard curve
were performed. The data collected for each amount for all three curves (i.e.
nine data points per amount) were averaged and replotted to yield a composite standard
curve for salvinorin A. Quantification of salvinorin A in plant
tissue samples. For each
sample, 20 microliter of the reconstituted methanol: acetone extract was
injected into the HPLC and the area of the peak due to 1 was integrated. This peak area was used to calculate the amount
of salvinorin A present in the tissue sample by applying the linear equation
obtained from the composite standard curve. RESULTS AND DISCUSSION Under the
isocratic chromatographic conditions, authentic salvinorin A (1) eluted
in approximately 8.0‑8.1 min (Fig. 1A). The analysis of the plant tissue
extracts described below allowed rapid elution of polar components, baseline
resolution of the analyte of interest, and a short analysis time (for examples,
see Fig. 1). 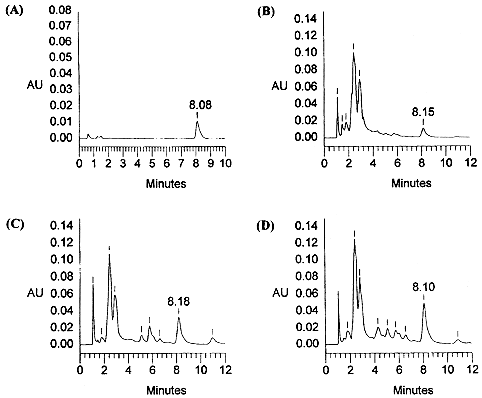 Figure
1. HPLC
chromatograms of an authentic sample (0.2551Ag) of salvinorin A (A), and
of representative Salvia divinorum tissue
extracts obtained from "Palatable" clone (Bret Blosser) (B),
from Cerro Rab6n clone (L. J. Valdés) (C), and from a seed grown plant
DS03 (D. J. Siebert) (D). In each case the retention time of the peak
representing salvinorin A is indicated. (For chromatographic protocol see
Experimental section). 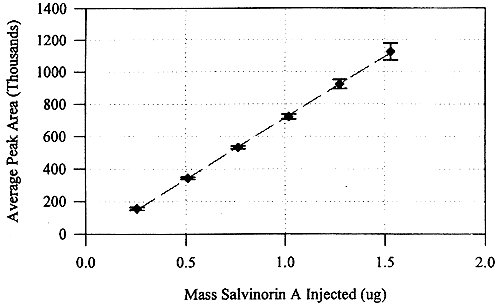 Figure 2. The composite standard calibration for the quantification of salvinorin A by HPLC (the error bars indicate ±1 standard deviation; the linear regression equation for the calibration curve is y= 759,334x ‑44,127). A composite
standard curve for 1 was compiled
from three individual determinations of the standard curve (Fig. 2). The
correlation coefficients for these three standard curves were 0.9997, 0.9997
and 0.9967. The coefficient of variation among the three curves was 5.9%. The
coefficients of variation for each injected amount across all three days ranged
from 1.7 to 5.7%. The peak
identified as salvinorin A (1) from
authentic standard solutions was observed in extracts of leaves and stem from S. divinorum. In order to examine the
concentration range of 1 found in various populations of S. divinorum, 20 different samples of
leaves were collected from cultivated plants in private collections as well as
endemic populations of the plant in Oaxaca. Representative chromatograms for
three different samples of S. divinorum are
shown in Fig. 1 Estimations were based on the average of two separate
injections. The determinations of the leaf content of salvinorin A showed a
broad range of values ranging from 0.89 to 3.70 mg/g dry weight (Table 1). Stem
material showed considerably lower values of 1 equivalent to
approximately 4% of the level found in leaves. 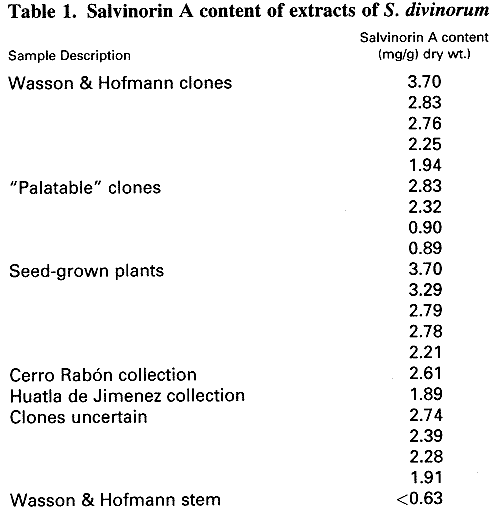 Since the genetic
heterogeneity of different populations of S.
divinorum is unknown, it is not possible to attribute the different levels
of 1 in these plants to any specific
environmental or genetic conditions. Four separate collections of the plant
which may represent distinct clones showed that variability in levels of 1 between clones is as great as the
variability between plants of the same clone grown in different locations.
These results are in contrast to earlier TLC analyses that suggested that there
were no appreciable differences in levels of salvinorin A among plants grown in
Michigan, Louisiana, or Mexico (Valdés, 1994). The Wasson and Hofmann clone
samples showed a range of 1.94 to 3.70 mg/g of 1 with an average value of
2.69 mg/g (±0.67, n = 5). The
"palatable" clone showed a range of 0.89 to 2.83 mg/g of 1 with an average of 1.73 mg/g (±0.99, n = 4). Two other samples from distinct
collections made in Oaxaca contained 2.61 and 1.89 mg/g of salvinorin A. An early report by
Epling and Játiva (1962) showing that S.
divinorum was most closely allied to S. concolor
Lamb ex. Benth led us to screen this species for salvinorin A content.
Ethnobotanical reports suggesting that Coleus
blumei was similarly used as a psychotropic by the Mazatec (Wasson, 1962)
caused us to screen one sample of this species as well for possible content of 1. HPLC analysis of the leaf extracts
of these two species did not show any salvinorin A present, nor was 1
found in leaf extracts of S. blepharophylla,
S. chiapensis, S. gregii var. San Isidro, S. leucantha, S. membranacea or S. recurva. In summary,
application of a novel HPLC methodology to quantify salvinorin A in S. divinorum leaves established the
typical levels of this compound in a number of samples of the plant. Further
screening of additional sage species for 1
may help elucidate the origins and taxonomic position of S. divinorum within the genus Salvia.
Additionally, this analytical assay can be utilized in screening for
salvinorin A in other S. divinorum plant
parts. ACKNOWLEDGEMENTS We
thank Dr. John R. Porter for advice on plant tissue culture and phytochemical
methods and for critical reading of the manuscript, Dr. Anil D'Mello for his
advice concerning the development of quantitative HPLC methodology, and Jeremy
Mihalov, Anne Giordano and Mila Denisova for their invaluable contributions. We
gratefully acknowledge PCPS for financial support, namely the Evelyn C. F. Ma
Memorial Research Fellowship (J.W.G.) and Summer Research Salary Support
(R.S.H.). REFERENCES Epling,
C. and Játiva‑M, C. (1962). A New Species of Salvia from Mexico. Bot. Mus, Leaflets, Harvard Univ. 20,
75‑76. McKenna,
D. J. (1996). Plant Hallucinogens: Springboards for Psychotherapeutic Drug
Discovery. Behav. Brain Res. 73, 109‑115. Ortega,
A., Blount, J. F. and Manchand, P. S. (1982). Salvinorin, a New Trans‑Neocleroclane
Diterpene from Salvia divinorum (Labiatae). J. Chem. Soc. Perkins Trans. 1,2505‑2508. Reisfeld,
A. S. (1993). The Botany of Salvia divinorum
(Labiatae). SIDA 15, 349‑366. Siebert,
D. J. (1994). Salvia divinorum and
Salvinorin A: New Pharmacologic Findings. J. Ethnopharmacology 43, 53‑56. Valdés
III, L. J. (1994). Salvia divinorum and
the Unique Diterpene Hallucinogen, Salvinorin (Divinorin) A. J. Psychoact. Drugs 26, 277‑283. Valdés
III, L. J., Diaz, J. L. and Paul, A. G. (1983). Ethnopharmacology of Ska Maria
Pastora (Salvia divinorum, Epling
& Jitiva‑M)., J. Ethnopharmacology 7, 287312. Valdés
III, L. J., Butler, W. M., Hatfield, G. M., Paul, A. G. and Koreeda, M. (1984).
Divinorin A, a Psychotropic Terpenoid, and Divinorin B from the Hallucinogenic
Mexican Mint Salvia divinorum. J. Org. Chem. 49, 4716‑4720. Valdés
III, L. J., Hatfield, G. M., Koreeda, M. and Paul, A. G. (1987). Studies of
Salvia divinorurn (Lamiaceae), a
Hallucinogenic Mint from the Sierra Mazateca in Oaxaca, Central Mexico. Econ. Bot. 41, 283‑291. Wasson,
R. G. (1962). A New Mexican Psychotropic Drug from the Mint Family. Bot. Mus. Leaflets, Harvard Univ. 20,7784. Parts
of this work were presented at the 1996 American Chemical Society Mid‑Atlantic
Regional Meeting, Student Award Symposium, The Chromatography Forum of Delaware
Valley, May 24, 1996, Villanova University, Villanova, PA, Abstract no. 363. Contract/grant
sponsor: PCPS, the Evelyn C. F. Ma Memorial Research Fellowship. Contrat/grant sponsor: PCPS, Summer Research Salary. |